Energy Storage Technologies
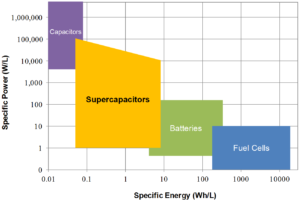
Batteries, fuel cells, capacitors, and supercapacitors are all energy storage devices. Batteries and fuel cells rely on the conversion of chemical energy into electrical energy. Capacitors rely on the physical separation of electrical charge across a dielectric medium such as a polymer film or an oxide layer.
Supercapacitors rely on the separation of chemically charged species at an electrified interface between a solid electrode and an electrolyte. Each type of device provides a different combination of power density and energy density, but only supercapacitors provide high power density and relatively high energy density.
Batteries
A battery is a device that transforms chemical energy into electric energy. All batteries have three basic components – an anode, a cathode, and an electrolyte – which determine their properties and performance. Batteries are broadly classified into primary and secondary.
Primary batteries are the most common and are designed as single use batteries, to be discarded or recycled after they run out. They have very high impedance, which translates into long life at low current loads. The most frequently used primary batteries are carbon-zinc, alkaline, silver oxide, zinc air, and lithium metals (like lithium manganese dioxide and lithium thionyl chloride).
Secondary batteries are designed to be recharged, and can be recharged up to 1,000 times depending on the usage and battery type. Very deep discharges result in a shorter cycle life, whereas shorter, shallower discharges will usually result in longer cycle life. The charge time varies from 1 to 12 hours, depending upon battery condition, Depth of Discharge (DoD), and other factors. Commonly available secondary batteries are nickel-cadmium, lead-acid, nickel-metal hydride, some lithium metal, and Li-ion. The limitations of secondary batteries include limited life, limited power, low energy-efficiency, and disposal concerns.
Fuel Cells
Like a battery, a fuel cell uses stored chemical energy to generate power. Unlike batteries, its energy storage system is separate from the power generator. It produces electricity from an external fuel supply as opposed to the limited internal energy storage capacity of a battery.
A typical fuel cell requires a large amount of extraneous control equipment like fuel pumps, cooling systems, fuel tanks and recirculators that makes them impractical for most portable applications. New developments like the small direct methanol fuel cell (DMFC) can do away with many of the extraneous systems, and offer some potential for use in portable devices. Fuel cells range in size from hand-held systems to megawatt power stations. Most large fuel cells operate at high temperatures (200ºC to 1000ºC), although proton-exchange membrane fuel cells (PEMFC) may be able to operate at room temperature.
Fuel cells operate most efficiently over a narrow range of performance parameters and at elevated temperature, rapidly becoming inefficient under high power demands. Fuel cells are often used in tandem with either batteries or supercapacitors to provide a high-energy, high-power combination. Use of catalyst metals, such as platinum, makes fuel cells an expensive proposition.
Capacitors
Capacitors use physical charge separation between two electrodes to store charge. They store energy on the surfaces of metallized plastic film or metal electrodes, with their capacitance being a function of the both dielectric medium and the overlapping surface areas of the electrodes. The dielectric medium acts as an insulator between the electrodes. Most configurations contain a layered arrangement, with a separation distance on the micrometer scale which is volumetrically inefficient.
Electrolytic capacitors rely on a layer of oxide material deposited on a metal surface. Here again, the thickness is on the micrometer scale and is very inefficient. Most capacitors can handle large voltages because they contain healing mechanisms that overcome the dielectric breakdown of the charge separation medium.
When compared to batteries and supercapacitors, the energy density of capacitors is very low – less than 1% of a supercapacitor’s, but the power density is very high. This means that capacitors are able to deliver or accept high currents, but only for extremely short periods, due to their relatively low capacitance.
Supercapacitors
Supercapacitors are very high surface area activated carbon capacitors that use a molecule-thin layer of electrolyte, rather than a dielectric, to separate the charge. The supercapacitor resembles a regular capacitor except that it offers very high capacitance in a small package. Energy storage is by means of static charge rather than the electrochemical process inherent to a battery. Supercapacitors rely on the separation of charge at an electrified interface that is measured in fractions of a nanometer, compared with micrometers for most polymer film capacitors.
In supercapacitors, the solution between the electrodes contains ions from a salt that is added to an appropriate solvent. The operating voltage is controlled by the breakdown voltages of the solvents, with aqueous electrolytes usually operating in the range of 0.5 – 1V, and organic electrolytes ranging from 2.1 – 3V.
There are three types of electrode materials suitable for the supercapacitor: high surface area activated carbons, metal oxides, and conducting polymers. The high surface area carbon electrode, also called and Electric Double Layer Capacitor (EDLC), is the least costly to manufacture, and is the most common. It stores the energy in the double layer formed near the carbon electrode surface.
The cycle life of a supercapacitor is virtually unlimited and their energy efficiency rarely falls below 90% when they are kept within their design limits. Their power density is higher than that of batteries, although their energy density is generally lower. However, unlike batteries, almost all of this energy is available in a reversible process.
Comparison Chart
The following table gives a brief summary of some critical properties of each technology. Because there are so many types with widely different properties, battery values are shown as a range.
| Property | CAP-XX Supercapacitors | Capacitors | Fuel Cells | Batteries |
| Charge/Discharge Time | Milliseconds to Seconds | Picoseconds to Milliseconds | 10 to 300 hrs. Instant charge (refuel). | 1 to 10 hrs |
| Operating Temperature | -40 to +85°C | -20 to +100°C | +25 to +90°C | -20 to +65°C |
| Operating Voltage | 2.3 to 2.75V | 6 to 800V | 0.6V | 1.25 to 4.2V |
| Capacitance | 100mF to 1500F | 10pF to 2.2mF | N/A | N/A |
| Life | 50,000+ hrs Unlimited cycles | >100,000 cycles | 1,500 to 10,000 hrs | 150 to 1,500 cycles |
| Weight | 1 g to 230g | 1g to 10kg | 20g to >5kg | 1g to >10kg |
| Power Density | 10 to 120 kW/kg | 0.25 to 10,000 kW/kg | 0.001 to 0.1 kW/kg | 0.005 to 0.4 kW/kg |
| Energy Density | 1 to 10 Wh/kg | 0.01 to 0.05 Wh/kg | 300 to 3,000 Wh/kg | 8 to 600 Wh/kg |
| Pulse Load | Up to 100A | Up to 1000A | Up to 150mA/cm2 | Up to 5A |